Lorem ipsum dolor sit amet, consectetur adipiscing elit. Proin nec erat sed lectus commodo vulputate…
Could intranasal delivery improve COVID-19 vaccines?
By Daisy Adamson
The COVID-19 pandemic resulted in the rollout of multiple effective vaccines including the Pfizer-BioNTech (BNT162b2) & Moderna (mRNA-1273) messenger RNA (mRNA) -based vaccines, and the Oxford/AstraZeneca (ChAdOx1 nCoV-19) & Janssen (Ad26.COV2.S) adenoviral vectored vaccines (1). The mRNA vaccines deliver SARS-CoV-2 Spike (S) protein mRNA within lipid nanoparticles (LNPs) (2), whilst DNA is delivered from adenoviral vectored vaccines (3). DNA is transcribed in the nucleus to mRNA. mRNA from both vaccines is translated by ribosomes, using tRNAs which deliver the appropriate amino acids to form the SARS-CoV-2 spike protein (4). Processing of spike protein results in antigen presentation through the major histocompatibility complex (MHC) Class I and II complexes. This stimulates activation of CD8+ and CD4+ T cells, respectively (5). There are two categories of antigen presenting cells (APCs). Professional APCs include dendritic cells, macrophages and B cells. Non-professional APCs are all nucleated cells that are not considered to be professional APCs. APCs express antigen which triggers an immune response, whilst professional APCs are involved in an enhanced response (6). Particularly, dendritic cells (DCs) which generate peptide-MHC complexes are transiently transduced directly with vaccine DNA (7) following adenoviral vectored vaccination. B cells (or B lymphocytes) recognising the intact S protein also undergo activation and proliferation in germinal centres and extrafollicular areas. Activated and proliferated B lymphocytes migrate to areas with stromal elements where their contact results in differentiation to plasma cells. These plasma cells produce a range of antibody isotypes, for example immunoglobulin A (IgA) and immunoglobulin G (IgG) (8). After vaccination, S-specific memory develops, triggering attack by memory T and B cells should an individual encounter S protein again via natural SARS-CoV-2 exposure (9)
As with many medicinal products, adverse effects are reported. For example, the Pfizer/BioNTech (BNT162b2) mRNA vaccine has induced allergic reaction and myocarditis in rare cases (10). For adenoviral vectored vaccines, vaccine induced thrombotic thrombocytopenia (VITT) is an extremely rare but potentially life-threatening condition whereby abnormally high platelet activation results in the formation of blood clots (11), and is associated with the AstraZeneca (ChAdOx1 nCoV-19) & Janssen (Ad26.COV2.S) vaccines. In the clinic, increased awareness of the condition and the development of more effective management pathways has reduced mortality rates by 90% (11). One hypothesis states that during VITT, the adenovirus binds platelet factor 4 (PF4) on platelets. The adenovirus/PF4 complex is recognised by B cells which in turn generate anti-PF4 antibodies (12). Enhanced platelet activation by anti-PF4 antibodies via Fc-gamma (Fcy) receptor IIA dependent platelet activation then gives rise to the clinical features of VITT (13).
In terms of efficacy, both adenoviral vectored and mRNA-based COVID-19 vaccines are successful. The Pfizer/BioNTech, Moderna, Janssen and AstraZeneca vaccines have reported effectiveness of 95%, 94.1%, 73.1% and 70.4%, respectively. Effectiveness relates to protection from SARS-CoV-2 disease compared to a placebo control group (14). In some contexts, the adenoviral vectored vaccines are favourable. The adenoviral vectored vaccines have been associated with lower overall mortality in clinical trials (15). Further, the Janssen and AstraZeneca adenoviral vectored vaccines can be stored between 3-6 times longer than the Moderna mRNA vaccine at temperatures between 2-8°C, whilst the Pfizer/BioNTech mRNA vaccine must be stored at -70°C (16). This is important when considering transportation and storage needs. Additionally, AstraZeneca estimates their adenoviral vectored vaccine costs $2.50 per dose, whilst the estimation of mRNA vaccines per dose is $15-$20 (17).
Sterilising immunity is the phenomenon whereby a pathogen is eliminated before it replicates in the host (18). The nasopharynx and oral mucosa of the upper respiratory tract are initially affected after SARS-CoV-2 entry. Prevention of the spread of the SARS-CoV-2 pathogen into the lower respiratory tract could therefore be controlled by mucosal immunity in the upper respiratory tract (19). This sterilising immunity may be generated by immunisation inducing SARS-CoV-2 antigen-specific immune responses in the upper respiratory tract (20). This strong mucosal immunity is challenging to generate (18), so vaccine technologies which induce better mucosal immunity are highly desirable.
Bharat Biotech International Limited (BBIL), has developed BBV154: an adenoviral vectored SARS-CoV-2 intranasal vaccine based on the chimpanzee adenovirus type 36 encoding a version of the SARS-CoV-2 spike protein. This vaccine induces strong mucosal and systemic immune responses on mice, rats, hamsters and rabbits preclinically. Encouragingly, BBV154 has now successfully completed phase 3 clinical trials in humans (21), followed by local licensure in India.
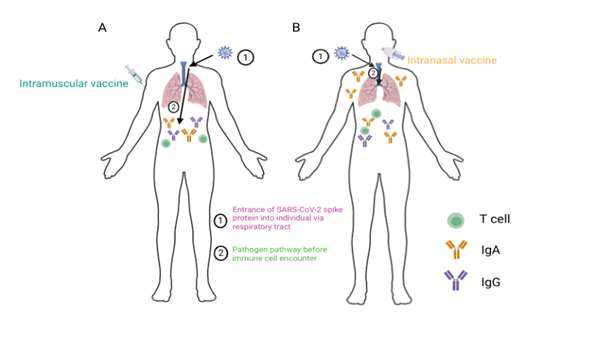
Figure 1 summarises immune responses induced by adenoviral vectored vaccines delivered via the intramuscular and intranasal routes in humans.

Figure 1: A schematic comparison of immune cell responses after intramuscular (IM, left) versus intranasal (IN, right) vaccination. The IM whole-inactivated Covaxin and IN BBV154 vaccines induce serum IgG and IgA responses against the SARS-CoV-2 spike protein (A, B). BBV154 but not Covaxin induces salivary IgA responses against the SARS-CoV-2 spike protein (B). BBV154 and Covaxin vaccination induces IFNγ secreting T cells against the SARS-CoV-2 spike protein (A, B). (21). Figure produced in BioRender.com.
IgG antibodies are present in the blood and extracellular fluid, whilst the IgA antibodies also reside in the mucosa, namely the intestinal and respiratory tracts (22). IgG antibodies rely on transport to the mucosal surfaces (23). Salivary IgA, residing in the respiratory tract, plays an important role in mucosal immunity within the lungs (24). This mucosal immunity has the potential for sterilising immunity following entry of a respiratory pathogen. The effector cytokine interferon gamma (IFNγ) released from T cells is involved in the removal of intracellular pathogens and the upregulation of MHC Class I and II (25) for CD8+ and CD4+ T cell activation.
Additionally, there is likely a reduced risk of VITT due to the nature of intranasal vaccine delivery (26). The adenoviral vectors used in vaccines are replication deficient (27) whilst Ad5 based adenoviral vectored vaccine administration in mice does not lead to Ad5 DNA in the spleen after intranasal delivery, an effect that is present after intramuscular delivery (26). Since VITT is thought to develop by the spread of adenovirus systemically via the bloodstream, the lack of adenoviral vector spread after intranasal delivery in comparison to intramuscular delivery highlights additional potential benefits for the delivery of these vaccines via the intranasal route.
Other benefits to intranasal vaccine delivery include needle-free administration (28), with the potential to significantly reduce needle related anxiety or vaccine-avoidance. Similarly, this easier administration method is more suitable for certain vaccine recipients e.g. elderly, children and those with HIV. Finally, if another global emergency were to occur like the COVID-19 pandemic, intranasal vaccination can be carried out by less skilled persons (29), increasing administrative efficiency and vaccine coverage.
In conclusion, intranasal vaccine delivery may solve multiple challenges, including those surrounding a lack of sterilising immunity, the induction of VITT, and administration related difficulties of intramuscular vaccination. Whilst the BBIL BBV154 vaccine is currently the only approved intranasal adenovirus-based vaccine against COVID-19, it is hoped that more intranasal vaccines will be developed and approved in future. These intranasal vaccines are highly promising candidates for contributing to profound improvements on human health by reducing illness severity and disease related mortality globally, especially in the context of respiratory pathogens.
References
This blog is adapted from a written report from a Professional Training Year placement at Cardiff University (October 2023 – September 2024).
6. Waithman, J., Moffat, J.M., Patterson, N.L., van Beek, A.E. and Mintern, J.D. 2014. Antigen Presentation. In: Reference module in biomedical sciences. Elsevier. doi: 10.1016/B978-0-12-801238-3.00118-5.
23. Borrok MJ, DiGiandomenico A, Beyaz N, Marchetti GM, Barnes AS, Lekstrom KJ, et al. Enhancing IgG distribution to lung mucosal tissue improves protective effect of anti–Pseudomonas aeruginosa antibodies. JCI Insight. 2018 Jun 21;
![BSGCT [Development]](https://cjamwebdemo.co.uk/wp-content/uploads/2024/12/bsgct-web-logo.png)


Comments (0)